In vivo imaging reveals transient microglia recruitment and functional recovery of photoreceptor signaling after injury
, , , , and
Significance
Microglia, the resident macrophages of the central nervous system, are critical for synaptic pruning and maintenance and for mitigating injury and neurodegeneration. Determining whether microglia–neuron interactions are beneficial in specific instances has been difficult, largely because of the local and transient nature of the interactions. Using simultaneous optical coherence tomography/scanning laser ophthalmoscopy (SLO) and adaptive optics SLO retinal imaging in mice, we show interactions of microglia and photoreceptors over time scales from seconds to months during injury, degeneration, and repair. In vivo optical assessment of photoreceptor signaling in a large neuronal field encompassing the injured area allows us to relate the time course of these microglia movements to that of the tissue remodeling and functional recovery.
Abstract
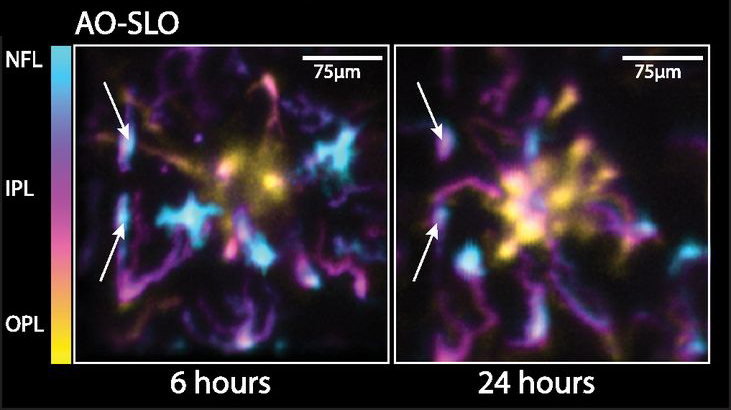
Microglia respond to damage and microenvironmental changes within the central nervous system by morphologically transforming and migrating to the lesion, but the real-time behavior of populations of these resident immune cells and the neurons they support have seldom been observed simultaneously. Here, we have used in vivo high-resolution optical coherence tomography (OCT) and scanning laser ophthalmoscopy with and without adaptive optics to quantify the 3D distribution and dynamics of microglia in the living retina before and after local damage to photoreceptors. Following photoreceptor injury, microglia migrated both laterally and vertically through the retina over many hours, forming a tight cluster within the area of visible damage that resolved over 2 wk. In vivo OCT optophysiological assessment revealed that the photoreceptors occupying the damaged region lost all light-driven signaling during the period of microglia recruitment. Remarkably, photoreceptors recovered function to near-baseline levels after the microglia had departed the injury locus. These results demonstrate the spatiotemporal dynamics of microglia engagement and restoration of neuronal function during tissue remodeling and highlight the need for mechanistic studies that consider the temporal and structural dynamics of neuron–microglia interactions in vivo.