Neuroinflammation
We study the inflammatory response to photoreceptor degeneration, specifically the activation of resident immune cells, called microglia, and, in some cases, the infiltration of monocytes across the blood-retinal barrier.
Microglia are yolk-sac derived macrophages that are normally highly ramified cells with dynamic processes that provide routine maintenance to synapses and monitor the CNS for signs of stress. In the retina, they predominantly reside in the synaptic layers but rapidly become ameboid and migrate to the outer retina in response to photoreceptor stress and degeneration.

Microglia (green) both in vitro (outer panels) and in vivo (inner panels) can be seen changing shape and migrating towards stressed and dying photoreceptors. Adapted from Levine et al., (2014) Vision Res. and Zawadzki et al.,(2015) Biomed Opt Express.
All rights reserved. Burns Lab, UC Davis.
In addition, we have shown that within 24 hours of photoreceptor stress, monocytes begin to extravasate from retinal vessels and differentiate into macrophages, creating a heterogeneous population of immune responders.
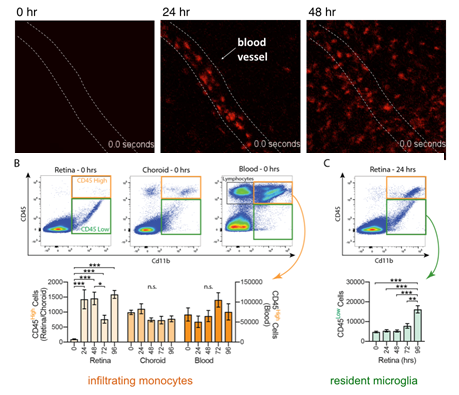
A. In vivo SLO imaging of RFP+ monocytes in the retina shortly after the onset of photoreceptor degeneration.
B-C, Quantification of infiltrating and resident cells in the retina during the time course of degeneration. Adapted from Karlen et al., (2018) J Neuroinflammation.
All rights reserved. Burns Lab, UC Davis.
We can distinguish activated microglia and infiltrating monocytes by looking at differences in gene expression using single-cell RNA sequencing, and then following these differences over time as degeneration proceeds.

Adapted from Ronning et al., 2019 Scientific Reports. All rights reserved. Burns Lab, UC Davis.